To enable quality assurance of the final product, it is important to develop PAT methods that generate meaningful measurements. This is a regulatory expectation for Continuous Manufacturing.
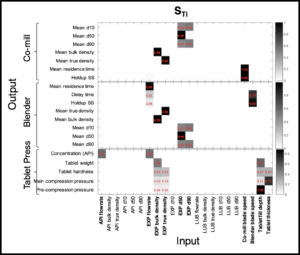
Our team uses multivariate methodologies to analyze spectroscopic and non-spectroscopic measurements, and implement effective approaches for real-time monitoring and quality assurance: blend composition and homogeneity, blend density, particle size, and product quality (including prediction of dissolution performance).
The next step is to implement and validate PAT performance on the continuous line. PAT chemometric models are developed and tested online, by running materials with known properties, to confirm PAT performance.
In these steps, our team uses manufacturing parameter dynamic information generated by the process equipment to implement effective approaches and controls for real-time monitoring and quality assurance of blend composition and homogeneity, blend density, particle size, and product quality (prediction of dissolution performance).
Once appropriate methods have been developed, the next step is to implement and validate performance on the continuous line. This is an opportunity to identify and address issues that emerge only during extended testing (like material filming on sensor surfaces). PAT chemometric models should also be tested online by running materials with known properties through the process and confirming the ability of the models to accurately predict these properties.